Hadlima is a prescription medication designed to treat a variety of chronic autoimmune conditions. Its active ingredient, adalimumab-bwwd, is a biosimilar to Humira, one of the most widely used biologic therapies in the world. By targeting tumor necrosis factor-alpha (TNF-α), Hadlima helps reduce inflammation and control symptoms in conditions such as rheumatoid arthritis, Crohn’s disease, ulcerative colitis, plaque psoriasis, and ankylosing spondylitis.
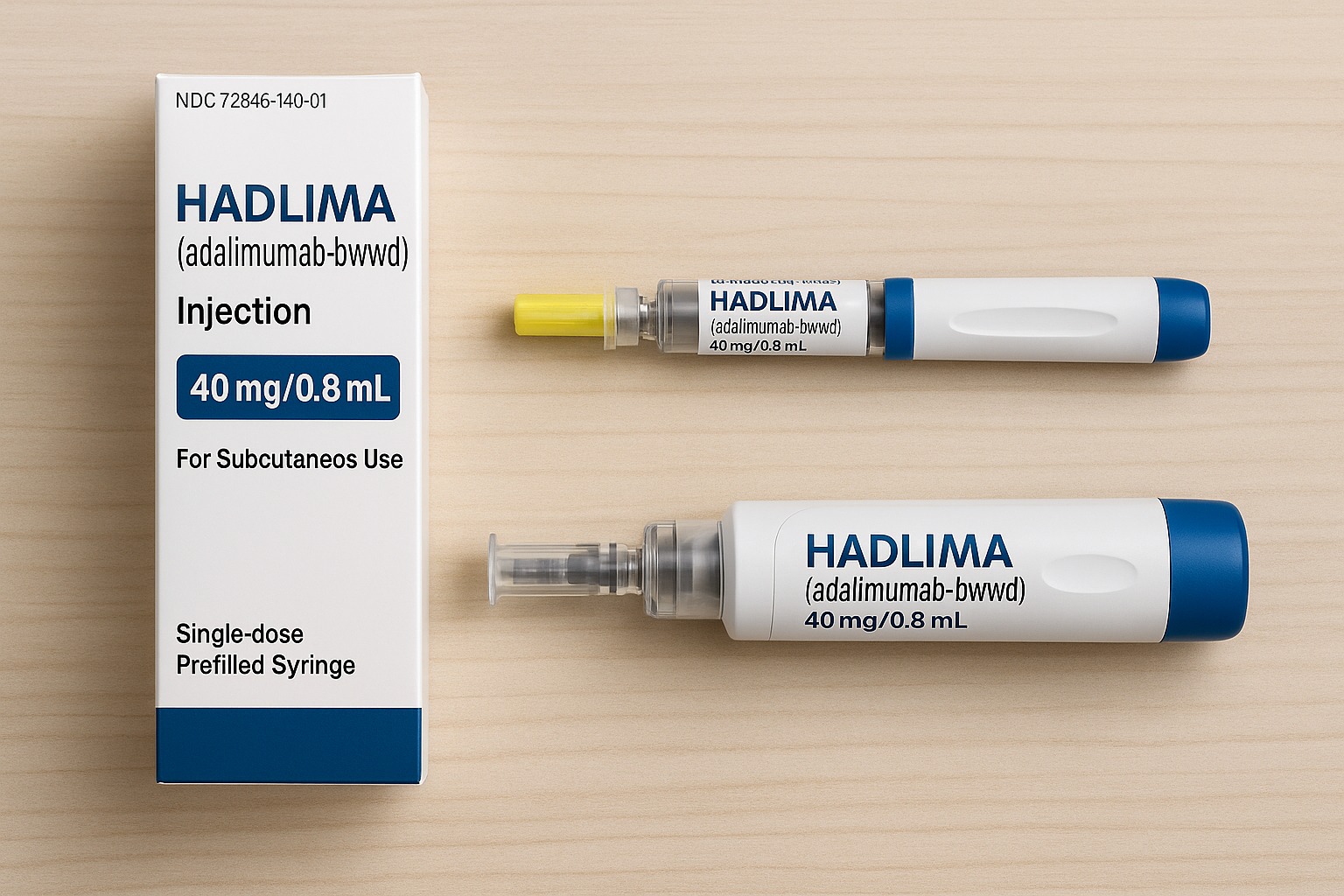
Hadlima is given by subcutaneous injection (under the skin), either through a prefilled syringe or an autoinjector pen that patients can use at home after proper training.
In this easy-to-read guide, you’ll learn what Hadlima is, how it works, when doctors prescribe it, common side effects, and answers to the most-searched questions about this Humira biosimilar.
Quick disclaimer: This article is for educational purposes only and not a substitute for professional medical advice. Always follow your doctor’s guidance when using Hadlima or any other medication.
What Is Hadlima?
Hadlima (adalimumab-bwwd) is an FDA- and EMA-approved biosimilar to Humira. Biosimilars are highly similar to an already approved biologic medicine (the “reference product”), with no clinically meaningful differences in safety, purity, or effectiveness.
Hadlima is manufactured by Organon and Samsung Bioepis and is available in two main delivery forms:
- Prefilled syringe
- Autoinjector pen (Hadlima AutoTouch™)
Both options are designed to make injections easier for patients who require long-term treatment.
How Does Hadlima Work?
Hadlima belongs to a class of medications called TNF-α inhibitors. TNF-α is a protein involved in inflammation. In autoimmune diseases, the body produces too much TNF-α, leading to painful swelling, tissue damage, and other symptoms.
By blocking TNF-α, Hadlima helps:
- Reduce inflammation
- Relieve pain and stiffness
- Improve physical function
- Slow or prevent joint and tissue damage
Common Uses of Hadlima
Doctors may prescribe Hadlima for several chronic inflammatory conditions in adults and children. These include:
- Rheumatoid arthritis (RA) – to reduce joint pain, swelling, and stiffness
- Psoriatic arthritis (PsA) – to improve joint symptoms and help control skin psoriasis
- Ankylosing spondylitis (AS) – for spinal inflammation and stiffness
- Crohn’s disease (CD) – to manage moderate to severe disease activity
- Ulcerative colitis (UC) – for moderate to severe cases not responding to other treatments
- Plaque psoriasis – for adults with moderate to severe skin involvement
- Juvenile idiopathic arthritis (JIA) – in certain pediatric patients
Your healthcare provider will decide if Hadlima is right for your condition.
Hadlima Dosage and Administration
The exact dose depends on your condition and doctor’s recommendations. General points include:
- Route: Subcutaneous injection (under the skin)
- Frequency: Often every two weeks (may vary by condition)
- Form: Prefilled syringe or autoinjector pen
- Training: Patients are usually trained by a healthcare provider to self-inject safely at home
If you miss a dose, follow your doctor’s instructions on when to take the next injection.
Side Effects of Hadlima
Like all medicines, Hadlima may cause side effects. Some are mild and temporary, while others can be more serious.
Common side effects include:
- Redness, itching, or pain at the injection site
- Upper respiratory infections (sinus infections, sore throat, cough)
- Headache
- Rash
Serious side effects (call your doctor right away):
- Signs of infection (fever, cough, shortness of breath, painful urination)
- Allergic reactions (hives, swelling, difficulty breathing)
- Nerve problems (numbness, tingling, vision changes)
- Blood disorders (unusual bruising or bleeding)
- New or worsening heart failure symptoms
Because Hadlima affects the immune system, it can increase the risk of infections. Regular monitoring and open communication with your healthcare team are important.
Warnings and Precautions
Before starting Hadlima, your doctor may check for conditions such as:
- Tuberculosis (TB) – testing is required before treatment
- Hepatitis B – screening may be recommended
- History of cancer or heart failure – may require careful evaluation
- Pregnancy & breastfeeding – use only if clearly needed, under medical supervision
Live vaccines should generally be avoided while using Hadlima.
Cost and Access
Hadlima was developed to offer a more affordable option compared to Humira. Prices can vary depending on insurance coverage, location, and pharmacy.
Many patients may qualify for savings programs, manufacturer assistance, or insurance support to reduce out-of-pocket costs. Ask your healthcare provider or pharmacist for details.
Frequently Asked Questions (FAQs)
Q1. Is Hadlima the same as Humira?
Hadlima is a biosimilar to Humira, meaning it works the same way and has no meaningful clinical differences.
Q2. How do I inject Hadlima?
Hadlima is given as an injection under the skin. Patients can use a prefilled syringe or an autoinjector pen after training.
Q3. How long does Hadlima take to work?
Some people notice improvements within 2 to 12 weeks, depending on their condition.
Q4. Is Hadlima safe long-term?
Hadlima has been tested in clinical trials and is considered safe when monitored by a doctor. Long-term use requires regular checkups.
Q5. Can I switch from Humira to Hadlima?
Yes, many patients switch from Humira to biosimilars like Hadlima. Your doctor will guide you through the transition.
Final Thoughts
Hadlima (adalimumab-bwwd) is an FDA-approved Humira biosimilar that offers patients a trusted and often more cost-effective option for managing chronic autoimmune diseases. With proper training, patients can self-administer Hadlima at home, making long-term treatment more manageable.
If you’re considering Hadlima, talk with your healthcare provider about whether it’s the right choice for your condition, and make sure to discuss side effects, monitoring, and financial support options.

Leave a Comment