Faslodex (generic name fulvestrant) is an estrogen-blocking cancer medicine given as a muscle injection. It’s used for hormone receptor–positive (HR+), HER2-negative advanced or metastatic breast cancer in postmenopausal women and adult men—either alone or together with CDK4/6 inhibitors (such as palbociclib, ribociclib, or abemaciclib) as prescribed by an oncologist.
Educational disclaimer: This guide is for general information only and isn’t medical advice. Treatment decisions for cancer must be made with your oncology team. Do not start, stop, or change any cancer medicine without your clinician’s direction.
What Is Faslodex?
Faslodex is an estrogen receptor (ER) antagonist and degrader (an SERD). Many HR+ breast cancers use estrogen signaling to grow. By blocking and breaking down the estrogen receptor, Faslodex helps slow or stop tumor growth.
Available formulation
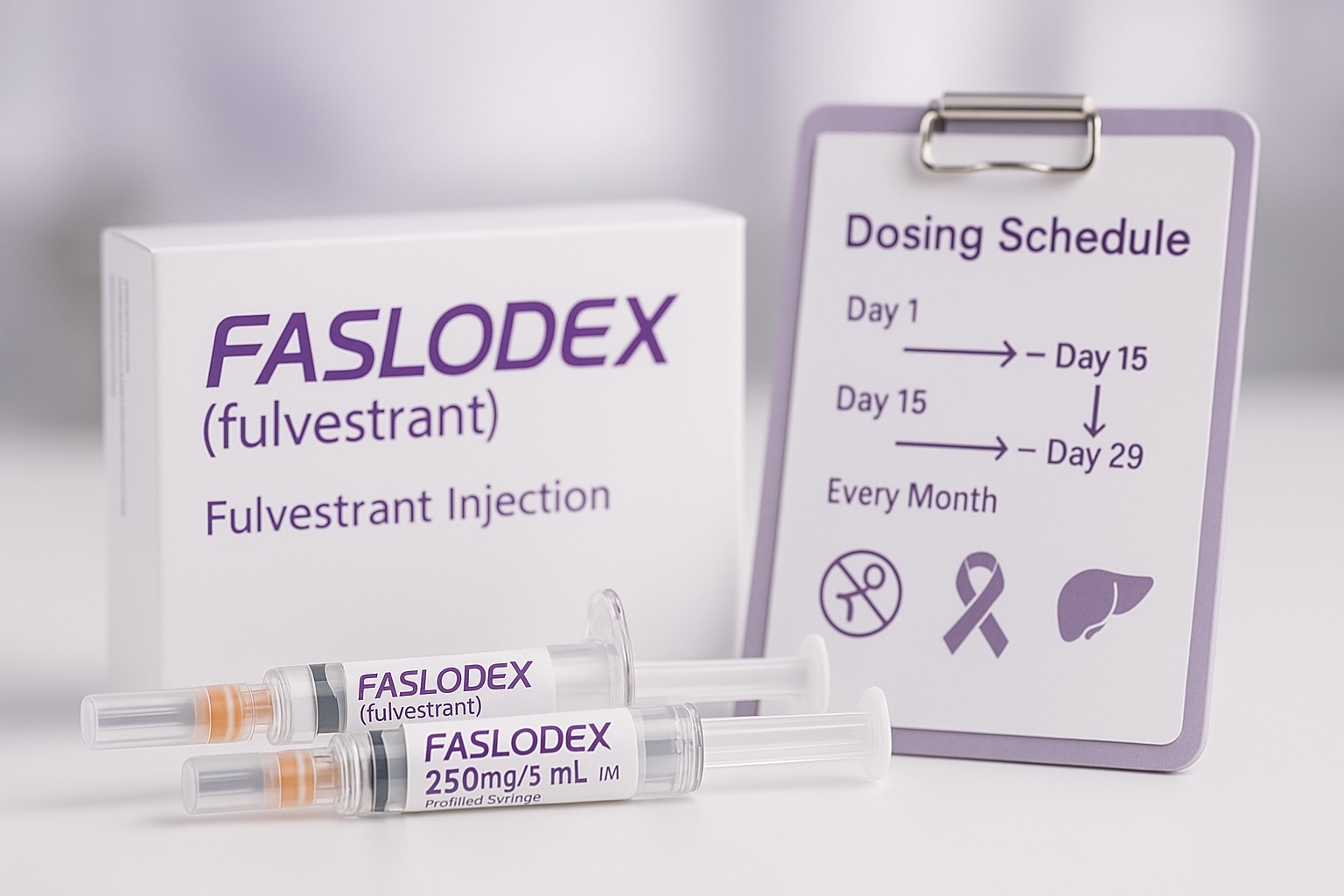
- Prefilled syringes containing 250 mg/5 mL fulvestrant (50 mg/mL).
A full dose is given as two injections (one in each buttock) for a total of 500 mg.
Faslodex is prescription-only and is administered by a trained healthcare professional.
How Does Faslodex Work?
- Binds to estrogen receptors (ERα) with high affinity, blocks estrogen from activating them.
- Accelerates receptor degradation, lowering the number of ERs in cancer cells.
- Shuts down ER-dependent gene signaling, which can reduce tumor cell survival and growth.
Because it removes and inactivates the target receptor, Faslodex can work even when other endocrine therapies (like tamoxifen) are no longer effective. Pairing Faslodex with CDK4/6 inhibitors targets the cell-cycle pathway at the same time, which can further delay disease progression in appropriate patients.
Common Uses
Your oncologist may use Faslodex for:
- First-line or later-line endocrine therapy for HR+, HER2- advanced or metastatic breast cancer in postmenopausal women and adult men
- In combination with a CDK4/6 inhibitor (palbociclib, ribociclib, or abemaciclib) for patients who are appropriate candidates
- After prior endocrine therapy (e.g., aromatase inhibitor or tamoxifen) when disease has progressed
Not for premenopausal patients without ovarian suppression. When needed, clinicians combine Faslodex with ovarian suppression (e.g., goserelin) to create a postmenopausal hormonal environment.
Dosage & Administration
Dosing is individualized. Fulvestrant is administered intramuscularly (IM) by a healthcare professional—do not self-inject.
Standard adult schedule (most patients):
- 500 mg IM on Day 1, Day 15, Day 29, then every month (every 28 days) thereafter.
Each 500-mg dose is split as two 250-mg injections, one into each gluteal muscle.
Hepatic impairment:
- For moderate liver impairment (Child-Pugh B), clinicians often use 250 mg on the same schedule (Day 1, 15, 29, then monthly).
- Severe liver impairment: use is generally not recommended.
Anticoagulation / bleeding risk:
- Because Faslodex is given IM, extra caution is required if you have low platelets or take blood thinners. Your team will weigh risks and may adjust timing or supportive care.
What to expect at the visit (step-by-step)
- Assessment: Brief symptom check (pain, hot flashes, injection-site issues, labs when needed).
- Positioning: You’ll typically stand or lie on your side for gluteal injections.
- Injection: Each syringe is given slowly (1–2 minutes) deep into the upper outer buttock.
- Aftercare: Light pressure and a small bandage; mild soreness is common.
- Scheduling: Book the next month’s dose; combination therapies may require additional monitoring (ECG, labs).
Side Effects
Common (often mild-to-moderate):
- Injection-site pain, swelling, or bruising
- Hot flashes, fatigue
- Nausea, decreased appetite, constipation
- Joint or muscle aches, back pain
- Headache
- Mild elevations in liver enzymes on blood tests
Less common but important—call your care team promptly:
- Serious liver problems: yellowing skin/eyes, dark urine, severe fatigue, right-upper-abdominal pain
- Allergic reactions: rash, itching, swelling of face/lips, trouble breathing
- Thromboembolic events: new chest pain, shortness of breath, leg swelling (seek urgent care)
Because Faslodex is an IM injection, localized discomfort is the most frequent issue. Applying a warm compress or gentle movement after the visit can help.
Warnings & Precautions
- Pregnancy & breastfeeding: Can harm an unborn baby. Do not use during pregnancy; use effective contraception during treatment and for the period your oncologist advises after the last dose. Avoid breastfeeding while on therapy.
- Liver disease: Requires careful monitoring; dose may be reduced in moderate impairment; avoid in severe impairment.
- Bleeding risk: Use caution with anticoagulants or low platelet counts due to IM administration.
- Premenopausal patients: Use only with ovarian suppression as directed by your oncologist.
- Driving/activities: If you feel dizzy or very tired after injections, wait until you feel well before driving or operating machinery.
Drug & Product Interactions
- CYP3A4 metabolism: Fulvestrant is metabolized mainly in the liver; however, clinically significant drug interactions are uncommon.
- Always provide a full list of prescriptions, OTC medicines, and supplements (including herbal products) to your oncology pharmacist.
- Anticoagulants/antiplatelets: Heightened bleeding risk comes from IM injection, not a direct drug interaction—your team will manage timing and precautions.
Faslodex vs. Other Endocrine Options
- Aromatase inhibitors (letrozole, anastrozole, exemestane): Lower estrogen production. Often used first-line; Faslodex may be used after progression or first-line in combination with a CDK4/6 inhibitor when appropriate.
- Tamoxifen: Blocks ER but does not degrade it. Faslodex degrades ER, which can help when tamoxifen or an AI no longer works.
- Oral SERDs (in development/various regions): Newer options aim to provide ER degradation by mouth; availability and indications vary.
- CDK4/6 inhibitors: When combined with Faslodex, these can extend time without disease progression in many patients who qualify, with added monitoring for blood counts, liver tests, and (for ribociclib) EKGs.
Choice depends on menopausal status, prior therapies, side-effect profile, tumor genetics, sites of disease, and insurance coverage.
Cost & Access
- Faslodex is a specialty oncology medicine administered in clinic. Costs vary by country, insurance, and setting.
- Ask your team about manufacturer patient-support programs, copay assistance, and financial counseling available through the clinic or cancer centers.
Special Section: Injection Details & Comfort Tips
- Why two shots? The standard 500-mg dose is supplied as two 250-mg syringes so the volume can be split between both gluteal muscles for comfort and absorption.
- Before your visit: Eat a light meal, hydrate, and wear clothing that allows easy access to the upper outer buttocks.
- After your visit: Gentle walking or stretching can reduce stiffness. If soreness persists, ask your team about acetaminophen or other options that are safe with your treatment plan.
Proper Care During Treatment
- Keep appointments on the Day 1/15/29 schedule, then every month.
- Report symptoms early: fevers, yellowing of eyes/skin, unusual bruising/bleeding, chest pain, shortness of breath, or severe injection-site reactions.
- Lifestyle support: balanced nutrition, activity as tolerated, sleep, and emotional support can improve quality of life during therapy.
- Fertility & contraception: Discuss family-planning goals. Reliable contraception is important during treatment and for the advised period after.
FAQs (12 Quick Answers)
- What is Faslodex used for?
HR+, HER2- advanced or metastatic breast cancer in postmenopausal women and adult men, alone or with a CDK4/6 inhibitor. - How is it given?
As two IM injections (one in each buttock) per dose—Day 1, Day 15, Day 29, then monthly. - Can I receive Faslodex at home?
It’s typically administered in a clinic by trained staff. - Do I need ovarian suppression?
Premenopausal patients generally need ovarian suppression alongside Faslodex; your oncologist will advise. - What are the most common side effects?
Injection-site pain, hot flashes, fatigue, nausea, joint aches, and mild liver-test elevations. - Will I lose my hair?
Hair loss is uncommon with Faslodex monotherapy. If combined with other agents, side effects may differ. - Is it safe with blood thinners?
Possible with precautions, but there’s injection-site bleeding risk. Your team will guide timing and monitoring. - Can I drink alcohol?
Small amounts may be acceptable for some, but because the drug is processed by the liver, discuss alcohol use with your oncologist. - What if I miss my monthly dose?
Call your clinic. You’ll usually receive it as soon as possible and reset your monthly schedule. - Is Faslodex used after an aromatase inhibitor stops working?
Yes—Faslodex is commonly used after AI progression, and often with a CDK4/6 inhibitor if appropriate. - Can I become pregnant on Faslodex?
Do not use during pregnancy. Use effective contraception and inform your team immediately if pregnancy occurs. - How quickly does it work?
Responses vary; oncologists assess with scans and labs every few months to evaluate benefit.
Final Thoughts (Best-Practice Summary)
- Targeted endocrine therapy: Faslodex degrades the estrogen receptor, offering a potent option for HR+ breast cancer, especially after prior endocrine therapy.
- Simple clinic schedule: Day 1/15/29, then monthly—plan ahead to stay on time.
- Safety first: Tell your team about liver issues, anticoagulants, or pregnancy plans; report new or worsening symptoms promptly.
- Teamwork matters: Optimal results come from close coordination with your oncology team, adherence to appointments, and early communication about side effects.

Leave a Comment