Fasenra (generic name benralizumab) is a prescription biologic for people 12 years and older with severe eosinophilic asthma that stays uncontrolled despite standard inhalers. It’s given as a small subcutaneous injection on a set schedule and can be administered in a clinic or, for many patients, at home with proper training (Fasenra Pen or prefilled syringe).
Educational disclaimer: This guide is for general information only and isn’t medical advice. Always follow your clinician’s instructions and the Medication Guide that comes with your prescription. Fasenra is not for sudden breathing problems, acute bronchospasm, or status asthmaticus—keep your rescue inhaler with you.
What Is Fasenra?
Fasenra is a monoclonal antibody that targets the IL-5 receptor alpha (IL-5Rα) on eosinophils, a type of white blood cell. Many people with severe asthma have an eosinophilic phenotype—high eosinophils that drive airway inflammation, frequent exacerbations, and reduced lung function. By selectively depleting eosinophils, Fasenra helps reduce asthma attacks, improves symptom control, and can lower oral steroid needs in appropriate patients.
Available formulations
- Fasenra prefilled syringe, 30 mg/mL (clinic or trained self-injection)
- Fasenra Pen (autoinjector), 30 mg/mL (trained self-injection option)
Both deliver the same dose into the thigh or abdomen (upper arm if someone else injects).
How Does Fasenra Work?
- Targets IL-5Rα on eosinophils. When Fasenra binds to the receptor, it flags eosinophils for removal.
- Enhances ADCC. Because the antibody is afucosylated, it binds more strongly to FcγRIIIa on natural killer (NK) cells, prompting antibody-dependent cell-mediated cytotoxicity (ADCC).
- Rapid depletion. Circulating eosinophils typically fall to near zero after the first doses, reducing eosinophilic airway inflammation.
What patients may benefit most?
People with severe asthma who, despite high-dose inhaled corticosteroids plus at least one controller (e.g., LABA), have elevated blood eosinophils and frequent exacerbations, or who require maintenance oral steroids.
Common Uses of Fasenra
- Add-on maintenance treatment for severe eosinophilic asthma in adults and adolescents (≥12 years).
- Not indicated for other conditions (e.g., COPD).
- Not for acute symptoms—do not use to treat a sudden asthma attack.
Dosage & Administration
Your exact plan is set by your clinician. Do not change or stop other asthma medicines (including oral steroids) unless directed—steroid doses are tapered gradually.
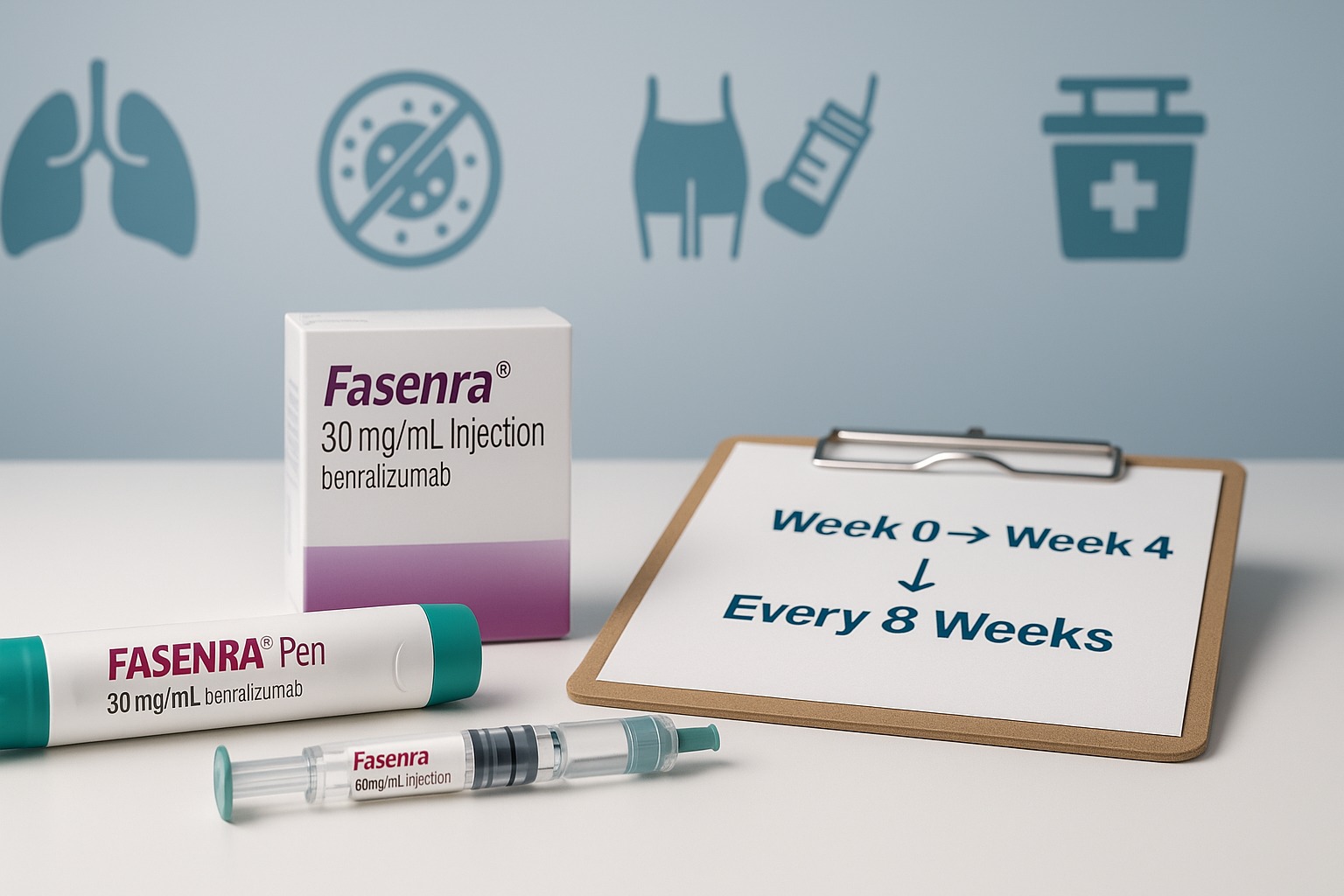
Standard adult/adolescent regimen
- 30 mg subcutaneously at Week 0, Week 4, then every 8 weeks thereafter.
Where & how it’s given
- Sites: Front of thigh or abdomen; upper arm if injected by a caregiver.
- Clinic vs home: Many start in clinic. After training and if appropriate, you may switch to at-home injections using the Fasenra Pen or prefilled syringe.
Step-by-step injection tips (after training)
- Store correctly (see storage below). Take one device out of the fridge and let it warm to room temperature as directed (usually ~30 minutes). Do not heat, do not shake.
- Inspect the device. Solution should be clear to slightly opalescent, colorless to slightly yellow; do not use if cloudy, discolored, or with particles, or if the device is damaged/expired.
- Prepare the site. Wash hands; clean skin with an alcohol swab; let dry.
- Inject as taught (pinch if needed, 90° or per device instructions).
- Dispose of the used device in an FDA-cleared sharps container.
- Record the dose date so you stay on the every-8-weeks schedule.
Missed dose
- Take it as soon as you remember, then resume the every-8-weeks interval from that date. If unsure, contact your clinic.
Side Effects
Common (often mild):
- Injection-site reactions (pain, redness, itching, swelling)
- Headache or sore throat
- Fever or back pain (less common)
Allergic reactions (rare but important):
- Hypersensitivity/anaphylaxis can occur hours to days after injection. Seek urgent care for hives, swelling of face/lips/tongue, trouble breathing, dizziness/fainting.
Parasitic (helminth) infections:
- Eosinophils help fight certain parasites. If you have a known helminth infection, it should be treated before starting Fasenra. If you become infected and don’t respond to treatment, your clinician may pause Fasenra until the infection clears.
Warnings & Precautions
- Not for sudden symptoms. Keep your rescue inhaler (e.g., albuterol) on hand.
- Steroid tapering: If you’re on oral or high-dose inhaled steroids, your prescriber will reduce slowly to avoid withdrawal or flare-ups.
- Helminth risk: As above—treat infections first; monitor exposure risk when traveling to endemic areas.
- Vaccines: Fasenra is not broadly immunosuppressive. Routine inactivated vaccines are generally okay. Discuss timing of live vaccines with your clinician.
- Pregnancy & breastfeeding: Human data are limited. If pregnant or planning pregnancy, discuss risks and benefits; ask about pregnancy exposure registries.
- Children: Approved for ≥12 years in many regions; follow local labeling.
Drug & Product Interactions
Fasenra does not rely on liver enzymes for clearance, so drug–drug interactions are uncommon. Still, always share an up-to-date medication list (prescriptions, OTC, supplements).
Key practical interactions
- Systemic steroids: Doses often change after response to Fasenra—never adjust on your own.
- Antiparasitics: May be needed if you’re at risk or infected with helminths.
- Other biologics: Combining biologics is uncommon—your specialist will decide.
Fasenra vs. Other Asthma Biologics
What sets Fasenra apart
- Mechanism: IL-5Rα blocker that depletes eosinophils via ADCC (Nucala/reslizumab block IL-5 itself; they reduce but don’t directly deplete cells).
- Dosing convenience: Every 8 weeks after the first two injections—less frequent than many alternatives.
- Phenotype focus: Strong option for eosinophilic asthma; works best when blood eosinophils are elevated.
Other options (selection depends on your biomarkers and comorbidities)
- Mepolizumab (Nucala) – IL-5 antibody; q4 weeks; also has indications for nasal polyps and EGPA.
- Reslizumab (Cinqair) – IL-5 antibody; IV infusion q4 weeks (adults).
- Dupilumab (Dupixent) – IL-4Rα blocker; eosinophilic or oral-steroid-dependent asthma; also for nasal polyps, atopic dermatitis, EoE.
- Tezepelumab (Tezspire) – TSLP inhibitor; works across phenotypes including low eosinophils; q4 weeks.
Choice is individualized—based on eosinophils, FeNO, allergies, nasal polyps, exacerbation history, steroid needs, dosing preference, and coverage/cost.
Cost & Prescription Status
- Fasenra is a specialty, prescription-only biologic.
- Cost varies by insurance, location, and setting (clinic vs home).
- Support programs and copay assistance may be available through the manufacturer (often referred to as Access 360/Fasenra 360). Ask your clinic or pharmacist to help you enroll.
Special Section: Pen vs Prefilled Syringe (and Clinic vs Home)
Fasenra Pen (autoinjector)
- Designed for at-home use after training.
- Simple push-button activation; hidden needle.
- Audible/visual cues to confirm dose completion.
Prefilled syringe
- Can be used in clinic or at home after training.
- Allows a slower manual push, which some patients prefer.
Clinic vs home
- Clinic: Monitoring, observation after early doses, and help with questions.
- Home: Convenient; keep to the every-8-weeks schedule; maintain sharps safety and proper storage.
Proper Care & Storage
- Refrigerate at 2–8 °C (36–46 °F) in the original carton; protect from light.
- Do not freeze or shake.
- When ready to use, allow to reach room temperature as instructed (about 30 minutes).
- If needed, most devices can be kept at room temperature (≤25 °C/77 °F) for a limited window—check your exact product insert and do not return to the fridge after warming.
- Keep out of reach of children. Dispose of used devices in a sharps container.
FAQs (12 Quick Answers)
- Who is Fasenra for?
People 12+ with severe eosinophilic asthma that remains uncontrolled despite optimized inhalers. - How fast does it work?
Some feel better within weeks; reductions in exacerbations and oral steroid use typically become clear over the first few months. - What’s the dosing schedule?
30 mg at Week 0, Week 4, then every 8 weeks. - Can I self-inject?
Many patients can, after training and clinician approval, using the Fasenra Pen or prefilled syringe. - Do I stop my inhalers?
No. Keep all asthma medicines unless your clinician adjusts them. Steroids are tapered slowly. - What are common side effects?
Injection-site reactions, headache, sore throat—usually mild and short-lived. - What serious reactions should I watch for?
Allergic reactions (hives, swelling, trouble breathing); rare but require urgent care. - Can I get vaccines on Fasenra?
Most inactivated vaccines are fine; ask about timing for live vaccines. - What if I have a parasite infection or travel to an endemic area?
Treat helminths before starting; if infected while on therapy, your clinician may pause treatment until resolved. - Will Fasenra help if my eosinophils are low?
Benefits are strongest with elevated eosinophils. Your specialist will check blood counts and other markers. - Is Fasenra weight-based dosing?
No. It’s a fixed 30 mg dose for eligible patients. - What if I miss my injection?
Take it as soon as you remember and resume the every-8-weeks cadence. Call your clinic if unsure.
Final Thoughts (Best-Practice Summary)
- Right fit: Fasenra is a focused option for severe eosinophilic asthma—especially if exacerbations persist despite high-dose inhalers.
- Simple schedule: After two loading doses, it’s every 8 weeks—a convenience edge for many.
- Stay prepared: Keep your rescue inhaler available; never use Fasenra for sudden symptoms.
- Safety first: Learn injection technique, know allergic-reaction signs, and treat helminths before/while on therapy.
- Team approach: Continue inhaled therapy, follow your asthma action plan, and attend regular follow-ups to track symptoms, exacerbations, and steroid needs.

Leave a Comment